This Alzheimer's drug was called a 'breakthrough,' so why did 3 people die?
Lecanemab was hailed by headlines as a breakthrough treatment for Alzheimer's. Now, with the death of a third patient, there are concerns about this medication.
Alzheimer's disease is very scary, so when it was announced that a new treatment can slow cognitive decline, there was great enthusiasm. However, a third death linked to the drug during clinical trials has raised fears regarding its safety.
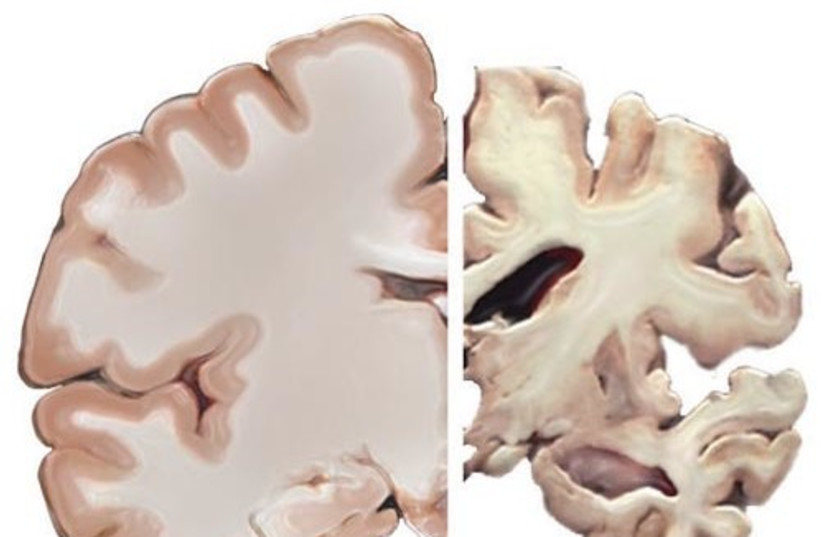
New medical records have shown that a 79-year-old Florida woman who took part in the trials died in mid-September after extensive brain swelling, bleeding and seizures. Neuroscientists who studied these records say that her death was likely caused by the drug lecanemab, which has been considered a breakthrough in the treatment of Alzheimer's.
"The brain swelling and the microhemorrhages … could be a serious side effect of the study medication," Leiden University neuroscientist and neurologist Ellis van Etten said, according to the website of the academic journal Science.
Japanese biotech firm Eisai Co., which makes the drug under the brand name Leqembi alongside US firm Biogen, didn't disclose the third patient's death at the annual Alzheimer's conference in San Francisco. Rather, the company instead revealed data from lecanemab's phase 3 trial that showed the slowed rate of cognitive decline in early Alzheimer's patients by an average of 27% over 18 months.
It is important to note that the third death's revelation came amid other reports of serious brain swelling and bleeding in the main clinical trial, as well as two other deaths earlier.
Eisai attributed these previous deaths and brain injuries to factors unrelated to their drug and didn't comment on the Florida woman's death, citing patient privacy concerns.
"All serious events, including fatalities, are reported to Eisai and considered in our evaluation of the study. This information is provided to the FDA [Food and Drug Administration] and other regulatory authorities," an Eisai spokesperson told Science.
According to them, when assessing a patient's death, age and medical condition must be taken into account. However, according to her medical records, the Florida woman had no apparent health problems aside from signs of early Alzheimer's.
Eiasi reported 13 deaths in a clinical trial featuring around 1,800 participants. According to Science, the company said that these deaths were expected given the participants' age and health conditions and explained that the number of deaths was similar in the lecanemab and placebo groups. However, the details of each death haven't been released, so scientists haven't been able to independently verify if the drug was responsible.
According to Science, lecanemab is one of several experimental Alzheimer's drugs that target amyloid beta, the protein that accumulates in the brains of Alzheimer's patients which many have theorized could be what causes memory loss and fatalities due to brain cell death.
However, drugs like this can come with a dangerous side effect: Amyloid-related imaging abnormalities (ARIA), which is brain swelling and bleeding, lecanemab trial investigator and Toronto Memory Program behavioral neurologist Dr. Sharon Cohen explained, according to CNN.
US FDA gives controversial Alzheimer's drug accelerated approval
However, just a month after the revelation of the third death, the US FDA gave accelerated approval to lecanemab, meaning it is approved for use in serious cases when there is an unmet medical need that the drug could help.
"Alzheimer's disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones," Billy Dunn, director of the Office of Neuroscience in the FDA's Center for Drug Evaluation and Research, said at the time in an FDA statement. "This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer's, instead of only treating the symptoms of the disease."
Months later, in March, Biogen revealed that the FDA would give lecanemab a priority review, which is needed for the drug to transition from accelerated approval to full approval. The results from this are due by July 6, 2023.
Jerusalem Post Store
`; document.getElementById("linkPremium").innerHTML = cont; var divWithLink = document.getElementById("premium-link"); if (divWithLink !== null && divWithLink !== 'undefined') { divWithLink.style.border = "solid 1px #cb0f3e"; divWithLink.style.textAlign = "center"; divWithLink.style.marginBottom = "15px"; divWithLink.style.marginTop = "15px"; divWithLink.style.width = "100%"; divWithLink.style.backgroundColor = "#122952"; divWithLink.style.color = "#ffffff"; divWithLink.style.lineHeight = "1.5"; } } (function (v, i) { });